- Augusta University
- Colleges & Schools
- Medical College of Georgia
- Neuroscience & Regenerative Medicine
- DNRM Microscopy Facility
DNRM Microscopy Facility
The DNRM Microscopy Facility houses two state-of-the-art Nikon A1R MP+ multiphoton/confocal microscopes, one Nikon C1 confocal microscope, two Nikon inverted fluorescent microscopes, and one Zeiss upright fluorescent microscope.
Useful links:
Confocal excitation & emission Resonant Scanners Intro to Multiphoton Microscopy
Tutorials Education & Resources
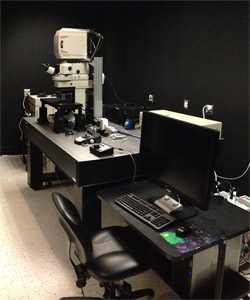
Nikon A1R MP+ Multiphoton/Confocal Microscope, System A
- Four high-sensitivity detectors for multiphoton imaging
- 7 visible laser lines (440, 458, 471, 488, 514, 561, 640nm) for confocal imaging
- MaiTai eHP DS ultrafast laser
- Full electrophysiology recording capabilities
- Fast imaging: 30 frames/second at 512x512 resolution, 420 frames/second maximum speed, high speed piezo-drive focus motor.
- Simultaneous photostimulation and imaging
- Best-in-class two-photon objective (25x, 1.1N.A., 2mm free working distance)

Nikon A1R MP+ Multiphoton/Confocal Microscope, System B
- Four high-sensitivity detectors for multiphoton imaging
- 4 visible laser lines (405, 488, 561, 640nm) for confocal imaging
- MaiTai eHP DS Ultrafast laser
- Fast imaging: 30 frames/second at 512x512 resolution, 420 frames/second maximum speed, high speed piezo-drive focus motor.
- Simultaneous photostimulation and imaging
- Best-in-class two-photon objective (25x, 1.1N.A., 2mm free working distance)

Nikon C1 confocal
- 3 visible laser lines (488, 543, 640nm) for confocal imaging
- Imaging workstation with deconvolution software

Nikon Eclipse TE-2000e Inverted Fluorescent Microscope
- Temperature controlled stage
- Camera for high speed imaging

Nikon Eclipse TE-300 Inverted Fluorescent Microscope
- Fitted with environmental controlled chamber for live cell imaging

Zeiss Axioplan2 Fluorescent Microscope
-
Multi-channel fluorescent imaging
-
Color CCD camera
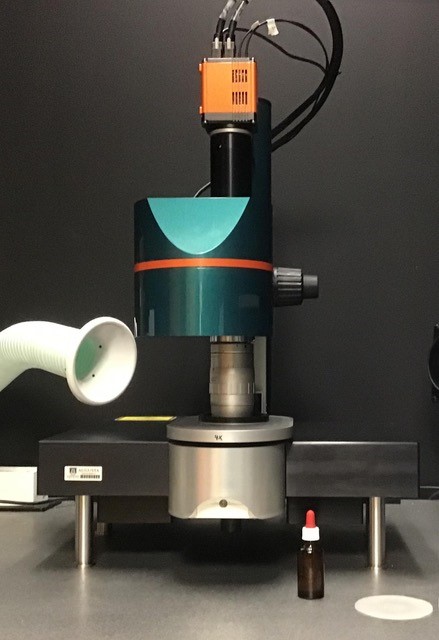
Ultramicroscope II Light-Sheet Microscope
-
-
-
-
- The Ultramicroscope II has an infrared laser and a second tunable laser for acquisition of up to three excitation channels (488/560/633). This system is capable of imaging samples up to 8x8cm in aqueous or organic media in Zoom Body or Super Plan configuration. In the Super Plan configuration with 12x objective, this system can acquire images with a maximal XY resolution of 0.217 microns/pixel. Resolution in the z-axis is determined by sample size, clearing efficacy, and laser configuration, but optical sections can be acquired at user-specified intervals for subsequent registration, segmentation, and high-throughput morphometric analysis. The departmental imaging facility also has two analysis workstations; one with Neurolucida 360 Studio for automated neuron tracing, dendritic spine and synapse detection, and a second with NeuroInfo software for registration onto the Allen Brain Atlas, automated segmentation, and cell detection (both from Microbrightfield). Users may schedule training sessions with Dr. Alexis M. Stranahan (astranahan@augusta.edu).
-
-
-