
Shared Resources
The Georgia Cancer Center supports shared research resources and facilities that provide important support to members of the Cancer Center and their collaborators.
The various resources offer access to state-of-the-art technology and computational support at an affordable cost. Training, consultation and support are also available. The Directors of the various cores are experts in the technologies that are offered in their respective Shared Resources.
Acknowledgment of all GCC Shared Resources is mandatory on publications resulting from consultation with personnel of the Shared Resouces. Significant involvement in the project, significant involvement in the interpretation of scientific data, involvement in the actual writing of a paper, use of original techniques, and/or novel experimental design by the facility staff are required to be acknowledged by co-authorship and acknowledgment of shared resource facility in accordance with all criteria set forth in Augusta University's hare policy on Authorship of Scholarly Activities. Decisions regarding authorship or acknowledgment should take place during the initial phase of the consultation process.
Jump to: Our Cores Meet our Associate Director Research News
Our Cores

BIOINFORMATICS
The Georgia Cancer Center's Bioinformatics Shared Resource offers state of the art bioinformatic support for analyses of genomic data and statistical support. In addition, the Resource manages the Georgia Cancer Center HPC for advanced computing.
BIOREPOSITORY
The Georgia Cancer Center's Biorepository provides a wide range of professional sample collection, annotation, and storage solutions. This Biorepository is also home to a state-wide resource, the Biorepository Alliance of Georgia for Oncology (BRAG-Onc), which functions to collect samples from 6 participating sites across the State to represent the diversity of cancer patients within the State and to enhance cancer research in Georgia.

BIOSTATISTICS
Biostatistics core (BC) is dedicated to supporting members of The Georgia Cancer Center in their investigative studies. Researchers will find expertise in planning, conducting, analyzing and reporting data relative to clinical trials as well as epidemiologic-, and population-based studies.

FLOW & MASS CYTOMETRY
The Georgia Cancer Center's Flow & Mass Cytometry Shared Resource provides investigators access to six flow cytometers, including two cell sorters and a variety of analytical instruments.
IMMUNE MONITORING
The Georgia Cancer Center's Immune Monitoring Shared Resource provides comprehensive immune monitoring services and standardized immune-based assays for basic, clinical and translational studies.

INTEGRATED GENOMICS
The Georgia Cancer Center's Integrated Genomics Shared Resource houses a complete Illumina Sequencing Facility, including Illumina HiSeq, NextSeq, MiSeq and an Ion Proton instruments. The Resource also supports Affymetrix array technologies and analytical support.
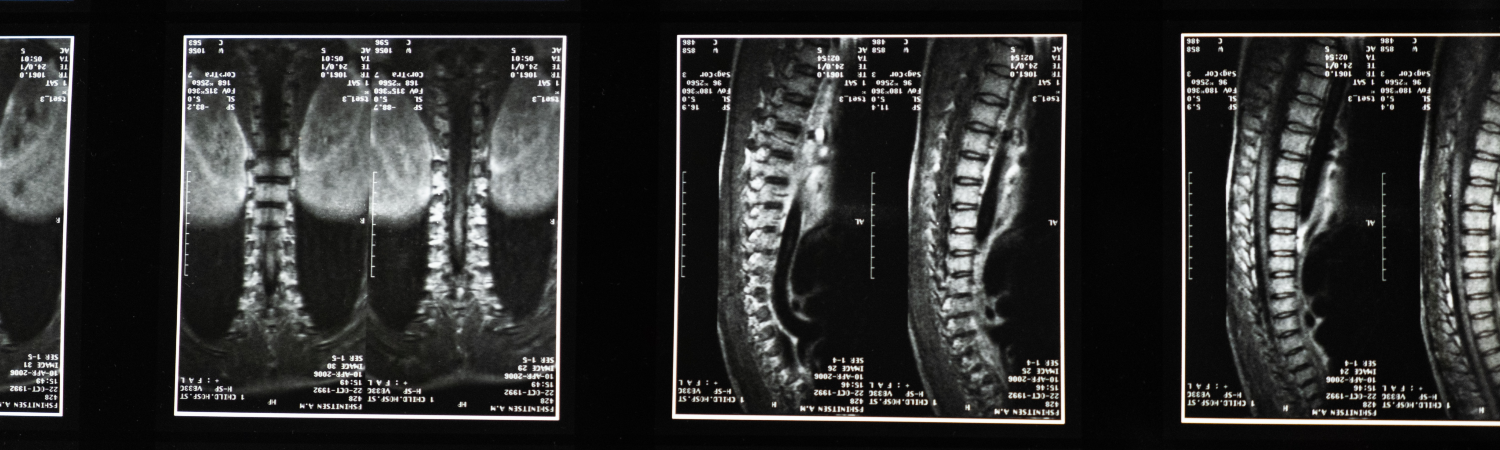
SMALL ANIMAL IMAGING
The Georgia Cancer Center's Small Animal Imaging Shared Resource provides magnetic resonance imaging (MRI) for small animals and offers whole-body and other imaging protocols specific to MRI such as quantitative MRI protocols.
Meet Our Associate Director

- Associate Director, Georgia Cancer Center Shared Resources
Klaus Ley is exploring the role our immune response plays in atherosclerosis, and his aim is to develop a vaccine and drugs that leverage human immunity to tackle the disease.
For more than two decades, Ley has applied his knowledge of immunology to understand the role that immune cells play in atherosclerosis. White blood cells swim in the blood, helping to protect the body from infection. To do their job, they must adhere to the blood vessel wall. In a chronic disease like atherosclerosis, this happens over and over again, eventually making the lumen narrower and the wall harder. So, instead of helping to solve a problem, the immune cells turn against the body — they actually hasten the atherosclerosis process.
As co-director of the Immunology Center of Georgia, Ley will build on his research while also recruiting candidates to establish a world-class immunology research center focusing on vaccines, cancer immunology and vascular immunology.
Research News

Revolutionary genome mapping tech targets childhood brain cancers
Revolutionary genome mapping tech targets childhood brain cancers
In Pictures: PaceDay 2025 celebrates cancer research
In Pictures: PaceDay 2025 celebrates cancer research
Cancer Center researcher targets colon cancer with a treatment fusion
Cancer Center researcher targets colon cancer with a treatment fusionThe Georgia Cancer Center at Augusta University is dedicated to reducing the burden of cancer in Georgia and across the globe through superior care, innovation, and education. Through unprecedented expansion, the Georgia Cancer Center is providing access to more first-in-the-nation clinical trials, world-renowned experts and life-saving options.
Follow the Georgia Cancer Center