Weiqin Chen, PhD
Professor

Phone: (706) 721-8706
Fax: (706) 721-7299
Email: wechen@augusta.edu
Office: CA-3094
Lab: CA-3093
Learn more about Dr. Chen's Research
Education and Training
Nanjing Agricultural University, Nanjing CHINA: BS in Microbiology, 1995
China Agricultural University, Beijing CHINA: MS in Microbiology, 1999
Michigan State University, East Lansing, MI USA: PhD in Molecular Genetics, 2005
Professional Experience
2005-2010 Postdoctoral Associate, Endocrinology & Metabolism with Dr. Lawrence Chan, Department of Medicine,
Baylor College of Medicine, Houston TX.
2010-2012 Instructor, Endocrinology & Metabolism, Diabetes & Endocrinology Research Center (DERC),
Baylor College of Medicine, Houston TX.
2012-2018 Assistant Professor, Department of Physiology, Augusta University, Augusta GA.
2018-present Associate Professor, Department of Physiology, Augusta University, Augusta GA.
Research Interests
The research in my laboratory is directed toward understanding mechanisms that regulate nutrient and energy metabolism, and how they become reprogrammed in obesity, lipodystrophy, and cardiometabolic diseases. We are specifically interested in the following areas: (1) Adipose biology and energy balance in obesity and lipodystrophy; (2) Cardiac substrate utilization in heart failure; (3) Lipid metabolism in liver pathophysiology. Using in vitro molecular/cellular biology approaches, a combination of genomics, transcriptomics, proteomics, CRISP/Cas9-mediated genome editing, and state-of-the-art in vivo mouse physiology analyses, we aim to define the pathological alterations of metabolic communication in diseases including obesity, diabetes, and their associated liver and heart diseases. Ultimately, we aim to identify targets and design therapeutics for these diseases.
Current Projects
To understand the mechanisms underlying adipose tissue development in obesity and lipodystrophy.
Obesity and its associated health comorbidities are a worldwide epidemic with serious economic and health burdens on society. Adipose tissue is a vital endocrine organ that plays a crucial role in developing obesity and various metabolic disorders. White adipocytes store excess energy in the form of triglycerides for future needs. By contrast, brown and beige (browning-in-white) adipocytes metabolize lipids and glucose to produce heat in a process known as nonshivering thermogenesis, which is crucial in systemic energy homeostasis and thermoregulation. Functional brown and beige adipose tissues are highly correlated with body mass index in adult humans and are either reduced or absent in obese and aged individuals and rodents. We are among the first to create the lipodystrophic Bscl2 global knockout mice and reveal the novel role of BSCL2 in regulating white and brown adipocyte differentiation, maintenance, and function, thus lipodystrophy and its associated comorbidities. We are now uncovering the molecular functions of posttranslational modifications (neddylation) and important GWAS-identified genes in adipose tissue development, obesity and lipodystrophy.
To investigate how altered lipid metabolism contributes to metabolic cardiomyopathy.
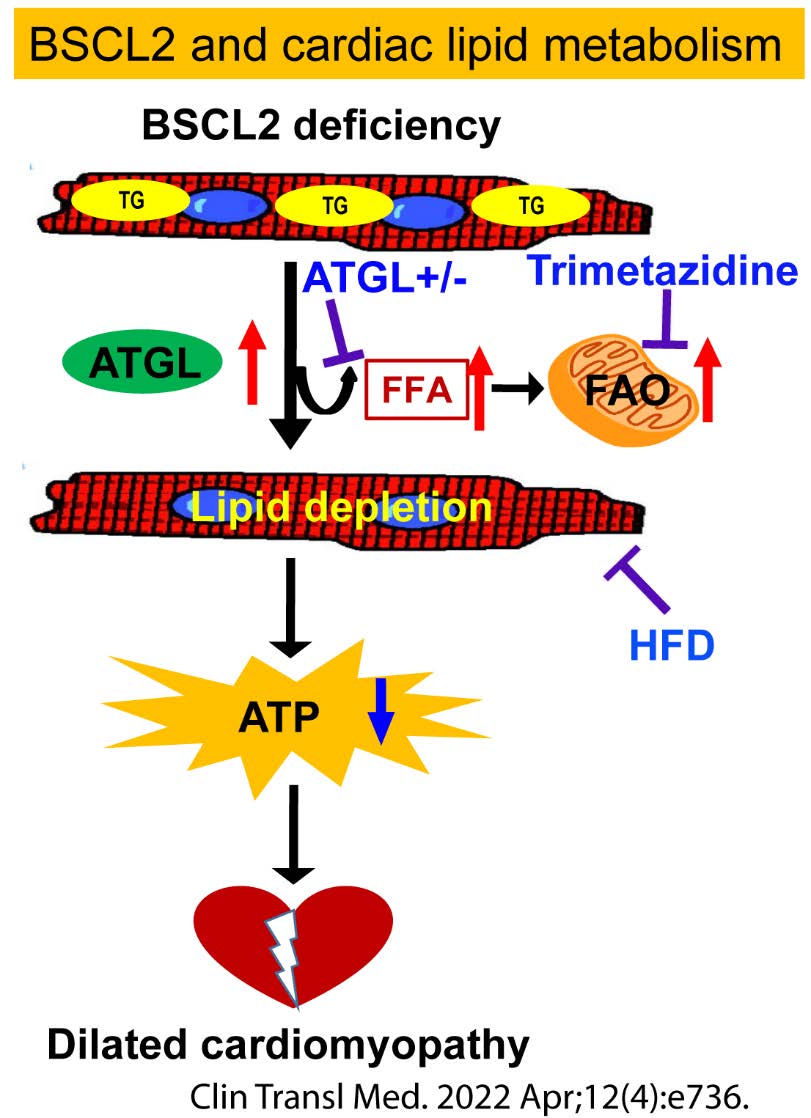
Metabolic perturbations underpin a wide variety of cardiomyopathies. In healthy humans, oxidation of FA is the dominant energy source for myocardial function and accounts for 60-70% of oxygen consumption. In obesity and diabetes, elevated FA supply chronically raises FA oxidation (FAO) in the heart, reducing cardiac efficiency and developing cardiac lipotoxicity. Heart TGs not only supply cardiomyocytes with FAs but are critical for the regulation of heart function. We have demonstrated the essential role of BSCL2 in regulating cardiac substrate utilization and function. We seek to advance our understanding of the novel players and mechanisms underlying cardiac TG lipolysis, mitochondrial function, and metabolic cardiomyopathy.
To probe the links between lipid metabolism and liver pathophysiology in obesity and diabetes.
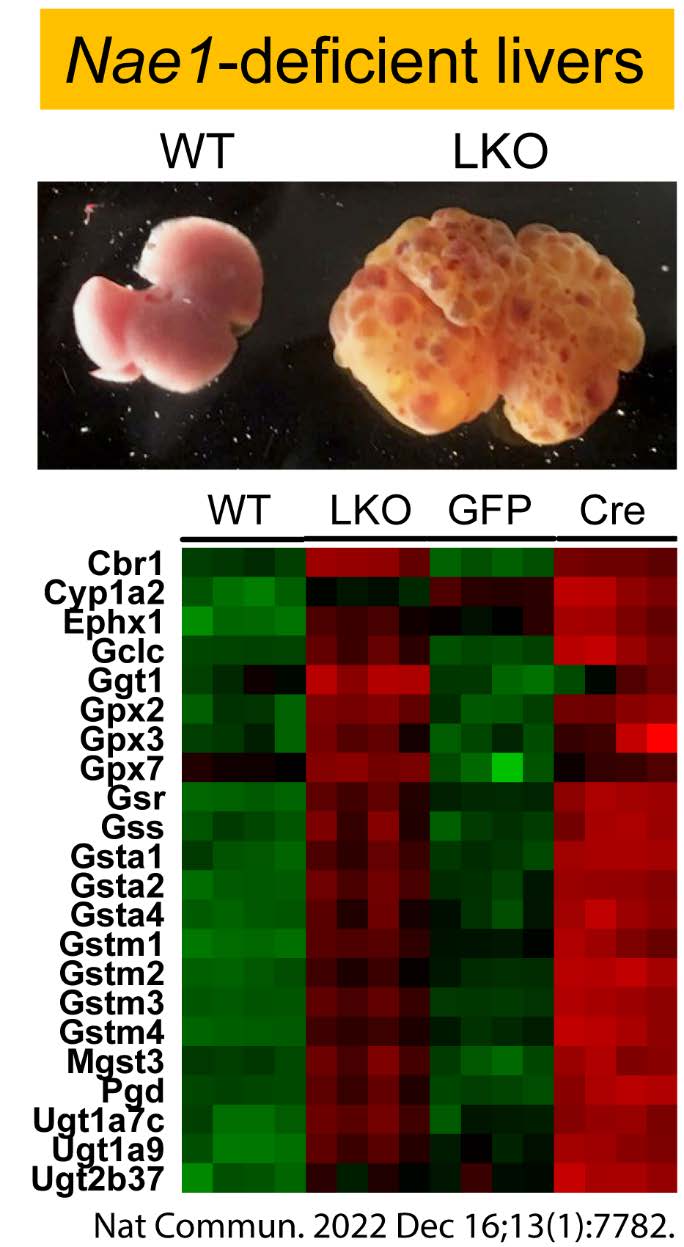
Nonalcoholic fatty liver disease (NAFLD), which ranges from NAFL to nonalcoholic steatohepatitis (NASH) and cirrhosis, is an underdiagnosed complication of obesity and type 2 diabetes (T2D). Up to 70% of patients with T2D have NAFLD, and people with diabetes have much higher rates of NASH, cirrhosis, and hepatocellular carcinoma (HCC) than the general, non-diabetic population. Hepatocellular death is a cardinal feature of NASH, which causes inflammation, fibrosis, cirrhosis and HCC. Therapeutic options for NAFLD in obesity and T2D are currently limited. A critical barrier to treatment is the lack of understanding of the molecular mechanisms that control hepatocellular death in NAFLD and NASH. We have demonstrated an essential role of NAE1-mediated neddylation in regulating liver development and function. We are interested in identifying novel player(s) that directly control lipotoxicity-induced hepatocellular death and facilitate the irreversible progression of NAFL to NASH in obesity and T2D.
Awards and honors
2017 Outstanding Young Basic Science Faculty Award from MCG Faculty Senate
2013 RNA-Seq Grant, Augusta University Cancer Center
2013 Eugenia Rosenberg Travel Award, Endocrine Society
2008 Postdoctoral Fellowship, American Heart Associatio
2006 Mentor Based Postdoctoral Fellowship, American Diabetes Association
2004 DuVall Award (Excellent graduate student scholarship), Michigan State University
Representative Publications
|
Zhou H, Fick K, Patel V, Hilton R, Kim H-W, Bagi Z, Weintraub NL, Chen W*. AGPAT3 Deficiency Impairs Adipocyte Differentiation and Leads to a Lean Phenotype in Mice. American Journal of Physiology Endocrinology and Metabolism . 2024 Jul 1;327(1):E69-E80. |
|
Xu C, Zhou H, Jin Y, Sahay K, Robicsek A, Liu Y, Dong K, Zhou J, Barrett A, Su H and Chen W*. Neddylation Deficiency in Hepatocytes Triggers Fatal Liver Injury via Inducing NF-κB-inducing Kinase. Nat Commun. 2022, Dec 16, 13(1):7782. PMCID: PMC9758150. |
|
Zhou H, Li J, Su H, Young M, Chen W*. BSCL2/Seipin Deficiency in the Heart Causes Energy Deficit and Heart Failure through Inducing Excessive Lipid Catabolism. Clin Transl Med 2022 Apr;12(4):e736. PMCID: PMC8982503. |
|
Zhou H, Xu C, Lee H, Yoon Y, Chen W*. Berardinelli-Seip Congenital Lipodystrophy 2/Seipin Determines Brown Adipose Tissue Maintenance and Thermogenic Programing. Mol Metab, 2020 Mar 4;36:100971. |
|
Zhou H, Lei X, Li J, Yun Y, Weintraub NL, Su H and Chen W*. Targeting ATGL to Rescue BSCL2 lipodystrophy and its Associated Cardiomyopathy. JCI Insight. 2019 Jun 11;5. pii: 129781. doi: 10.1172/jci.insight.129781. |
|
Tang J, Zhou H, Sahay K, Xu W, Yang J, Zhang W*, Chen W*. Obesity-associated Family with Sequence Similarity 13, Member A (FAM13A) is dispensable for adipose development and insulin sensitivity. Int. J Obesity (Lond). 2018 Oct 9. doi: 10.1038/s41366-018-0222-y. |
|
Xu W, Zhou H, Xuan H, Wang G*, Chen W*. Novel Metabolic Disorders and the Pathogenesis of Insulin Resistance in Skeletal Muscle of Lipodystrophic Bscl2/Seipin Deficient Mice. Mol. Cell. End. 2018; Aug 15. 411:207-13. PMID: 25958046. |
|
Zhou H, Black SM, Benson TW, Weintraub NL, Chen W*. Berardinelli-Seip Congenital Lipodystrophy 2 (BSCL2)/SEIPIN is not required for
Brown Adipogenesis but Regulates Brown Adipose Tissue Activation, Development and
Function. Mol Cell Biol. 2016 Jul 14;36(15):2027-38. PMID: 27185876. |
|
Zhou H, Lei X, Benson T, Mintz J, Xu X, Harris RB, Weintraub NL, Wang X, Chen W*. Berardinelli-Seip congenital lipodystrophy 2 regulates adipocyte lipolysis, browning, and energy balance in adult animals. J Lipid Res. 2015 Oct;56(10):1912-25. PMID: 26269358. Highlighted in ASBMB Today, 2015; 14(10):11. |
|
Lei X, Callaway M, Zhou H and Chen W*. Obesity associated Lyplal1 gene is regulated in diet induced obesity but not required for adipocyte differentiation. Mol. Cell. End. 2015; Aug 15. 411:207-13. PMID: 25958046. |
|
Chen W*, Zhou H, Saha, P, Li L and Chan L. Molecular mechanisms underlying fasting modulated liver insulin sensitivity and metabolism in male lipodystrophic Bscl2/Seipin-deficient mice. Endocrinology. 2014 Nov;155(11):4215-25. PMID: 25093462. |
|
Chen W*, Zhou H, Liu S, Fhaner CJ, Gross BC, et al. Altered Lipid Metabolism in Residual White Adipose Tissues of Bscl2 Deficient Mice. PloS ONE 2013 8(12): e82526. doi:10.1371/journal.pone.0082526. PMID: 24358199. |
|
Chen W, Chang B, Wu X, Li L, Sleeman M and Chan L. Inactivation of Plin4 downregulates Plin5 and reduces cardiac lipid accumulation in mice. Am. J. Physiol. Endocrinol. Metab. 2013 Apr;304(7):E770-9. |
|
Chen W, Chang B, Saha P, Hartig SM, Li L, Reddy VT, Yang Y, Yechoor V Mancini M, Chan L. Berardinelli-Seip Congenital Lipodystrophy-2 (BSCL2)/Seipin is a Cell Autonomous Regulator of Lipolysis Essential for Adipocyte Differentiation. Mol Cell Biol. 2012 Mar;32(6):1099-111. |
|
Chen W, Chang B, Li L and Chan L. Pnpla3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology 2010 Sep; 52(3): 1134-42. Comment by Farrell GC, Hepatology. 2010 Sep;52(3):818-21 |
|
Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L. The Human Lipodystrophy Gene Product BSCL2/Seipin Plays a Key Role in Adipocyte Differentiation. Endocrinology 2009 Oct; 150(10): 4552-61. |
We are actively recruiting Research Assistants, Undergraduates, Graduate students, and Postdoctoral fellows. Please contact Dr. Weiqin Chen at wechen@augusta.edu if you are interested.
LAB PERSONNEL
 Hongyi Zhou, PhD
Hongyi Zhou, PhD
Assistant Research Scientist
Pictured from left to right:
Hongyi Zhou, PhD
(Ast Research Scientist)Carl Whitehead (Research Ast)
Gavin Feinberg (Undergrad Student)
Weiqin Chen, PhD
ProfessorMousumi Baruah
Postdoctoral FellowXuelei Zhao
Temporary Paraprofessional