- Augusta University
- Colleges & Schools
- Medical College of Georgia
- Physiology
- Faculty | Physiology
- Christopher Waters, PhD
Christopher Waters, PhD

Professor, Physiology
706-721-7859
chrwaters@augusta.edu
Office: CA-2096| Lab: CA - 2095
706-721-7299
Lab Personnel

Emily Huffman
- MD/PhD Student
Education & TrainingAcademic AppointmentResearch InterestsAwards & AccomplishmentsPublications
Education
|
University of Tennessee at Chattanooga |
B.S.E. |
08/1985 |
Chemical Engineering |
|
University of Miami (FL) |
M.S. |
06/1987 |
Biomedical Engineering |
|
Vanderbilt University |
Ph.D. |
08/1991 |
Biomedical Engineering |
|
Vanderbilt University |
Postdoctoral |
06/1992 |
Biomedical Engineering |
Academic Appointments
Academic Appt.
- 2024-present
Professor, Department of Physiology, Medical College of Georgia, Augusta University
Academic Appt.
- 2024-present
Research Physiologist/Principal Investigator, Augusta Veterans Administration Healthcare System, Augusta, GA.
Academic Appt.
- 2017-2024
Dr. Donald T. Frazier Professor, Department of Physiology, College of Medicine, University of Kentucky, Lexington, KY.
Academic Appt.
- 2017-2024
Professor of Physiology, Saha Cardiovascular Research Center, College of Medicine, University of Kentucky, Lexington, KY.
Academic Appt.
- 2019-2024
Director, Kentucky Research Alliance for Lung Disease (K-RALD), University of Kentucky, Lexington, KY.
Academic Appt.
- 2023-2024
Research Physiologist/Principal Investigator, Lexington Veterans Administration Healthcare System, Lexington, KY.
Academic Appt.
- 2016-2017
Interim Chair, Department of Physiology, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 2005-2017
Professor of Physiology, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN
Academic Appt.
- 2007-2016
Vice Chair of Physiology, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN
Academic Appt.
- 2006-2007
Interim Chair, Department of Physiology, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 2006-2017
Professor of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 2012-2017
Professor of Orthopaedic Surgery and Biomedical Engineering, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 2001-2012
Assistant Professor of Biomedical Engineering and Imaging, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 2000-2017
Adjunct Faculty, Department of Biomedical Engineering, The University of Memphis, Memphis, TN.
Academic Appt.
- 2004-2006
Associate Professor of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, College of Medicine, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 1999-2005
Associate Professor of Physiology (with tenure), College of Medicine, The University of Tennessee Health Science Center, Memphis, TN.
Academic Appt.
- 1992-1999
Assistant Professor of Biomedical Engineering (Joint Appointment, 1996), Department of Biomedical Engineering, Northwestern University, Evanston, IL.
Academic Appt.
- 1992-1999
Assistant Professor of Anesthesiology, Department of Anesthesiology, Northwestern University School of Medicine, Chicago, IL.
Academic Appt.
- 1998-1999
Assistant Professor of Medicine, Department of Medicine, Northwestern University School of Medicine, Chicago, IL.
Academic Appt.
- 1993-1999
Member, Feinberg Cardiovascular Research Institute, Northwestern University School of Medicine, Chicago, IL.
Research Interests
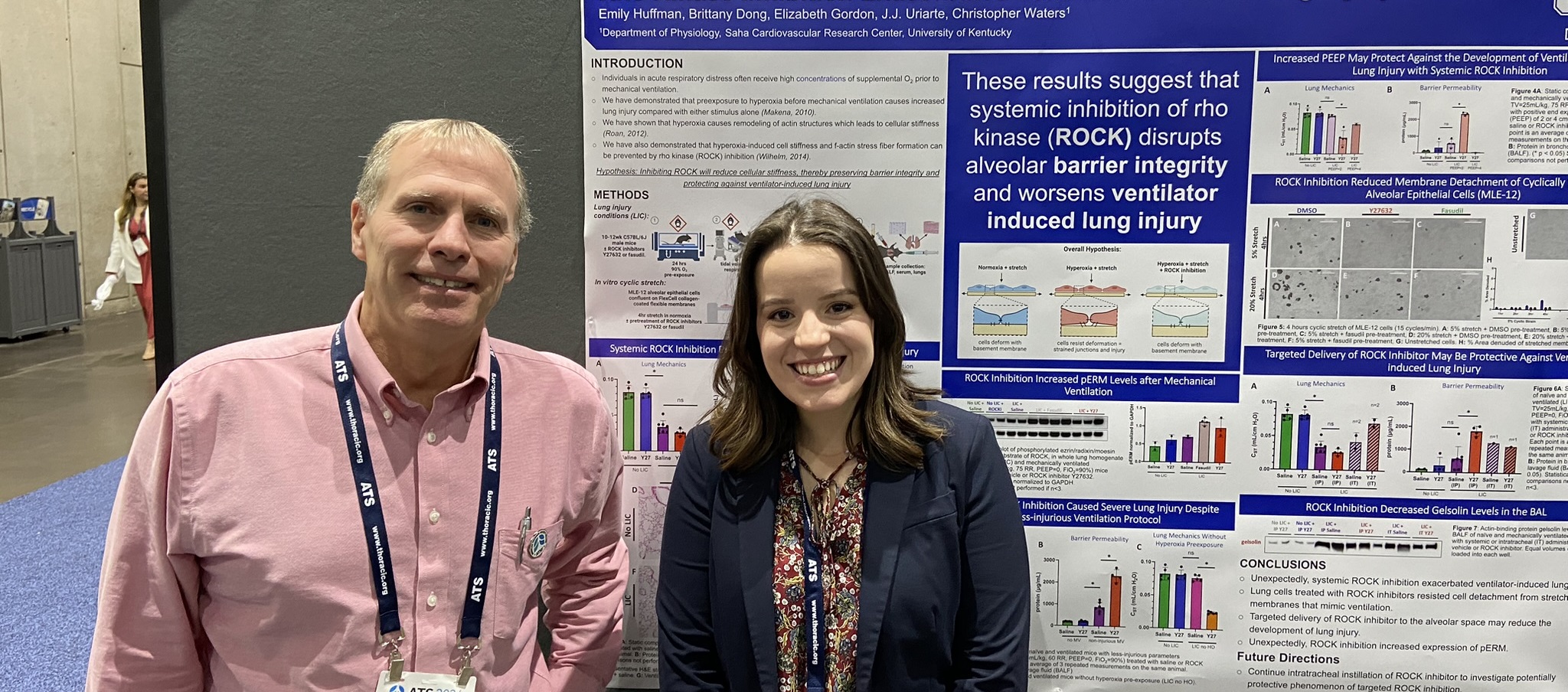
The Waters lab focuses on diseases of the lung with a focus on acute lung injury and pulmonary fibrosis and the role of mechanobiology. Patients with acute respiratory distress syndrome (ARDS) are placed on mechanical ventilators to improve oxygenation, but the ventilator may cause additional injury to the lungs due to either overdistention or airway collapse and reopening. Clinical trials have demonstrated a substantial reduction in mortality in ARDS patients when ventilation strategies are used that reduce overdistention (lower tidal volumes) and minimize airway collapse and reopening (positive end expiratory pressure). The lung is a mechanically dynamic organ, and cells in the lung are subjected to shear stress due to fluid flow, tensile and compressive forces due to respiratory motion, and normal forces due to vascular or airway pressure. High tidal volume mechanical ventilation in injured lungs induces mechanical stresses that increase injury to the lung epithelium, stimulate inflammatory responses, and decrease repair mechanisms. In addition, pulmonary fibrosis results in changes in mechanical properties of the lungs that profoundly impacts gas exchange. We are focusing on the mechanisms by which mechanical forces contribute to lung injury, inhibit wound healing of lung epithelial cells, and stimulate inflammation. We are examining signaling pathways related to injury and repair, pulmonary fibrosis, cell migration and wound healing, inflammasome activation, cytoskeletal remodeling, stimulation of reactive oxygen species, cytokine secretion, and regional variations in cellular tension. In addition we are examining lung injury in vivo and the effects of exposure to high levels of oxygen (hyperoxia). My research seeks to elucidate the molecular mechanisms of lung injury, repair, and remodeling, and to develop approaches to reduce lung disease.
Awards & Honors
- Chair, Respiration Section, American Physiological Society, 2023-present.
- Chair-elect/Chair, Program Committee for Respiratory Cell and Molecular Biology Assembly of the American Thoracic Society International Conference Committee, 2020-2022.
- American Thoracic Society Fellow (ATSF), 2022.
- Fellow of the American Physiological Society (FAPS), 2019.
- Member, Pulmonary Medicine Merit Review Board, Department of Veterans Affairs, 2015-2020.
- Editorial Board, American Journal of Physiology: Lung Molecular and Cellular Physiology, 2006-present.
- Editorial Board, American Journal of Respiratory and Critical Care Medicine, 2010-present
- Editorial Board, American Journal of Respiratory Cell and Molecular Biology, 2012-2022
- Editorial Board, Physiological Reports, 2013-2018; 2020-present.
- Experimental Biology Meeting, Joint Program Committee for the Respiration Section of the American Physiological Society, 2011-2015.
- Star Reviewer, American Journal of Physiology: Lung Cellular and Molecular Physiology, 2015.
- Member NIH Study Section Lung Injury, Repair, and Remodeling 2005-2009 (co-chair 06/08, 10/08)
- American Lung Association National Research Grant Review Committee, 2002-2004
- American Physiological Society Joint Program Committee Representative for the Respiration Section (Experimental Biology), 2011-2015.
- Giles F. Filley Memorial Award for Excellence in Respiratory Physiology and Medicine (American Physiological Society) 1998
Publications
Immanuel, C.N., B. Teng, B.E. Dong, E.M. Gordon, C. Luellen, B. Lopez, J. Harding, S.A. Cormier, E.A. Fitzpatrick, A. Schwingshackl, and C.M. Waters. Two pore potassium channel Trek-1 (K2P2.1) regulates NLRP3 inflammasome activity in macrophages, Am. J. Physiol. Lung Cell. Mol. Physiol . 326: L367-L376, 2024.
Conroy, L.R., H.A. Clarke, D.B. Allison, S.S. Valenca, Q. Sun, T.R. Hawkinson, L.E.A. Young, J. Juras, J.E. Ferreira, A.V. Hammonds, J.B. Dunne, R.J. McDonald, K.J. Absher, B.E. Dong, R.C. Bruntz, K.H. Markussen, W.J. Alilain, M.S. Gentry, J. Liu, P.M. Angel, C.M. Waters*, and R.C. Sun*. Spatial metabolomics reveals glycogen as an actionable target for pulmonary fibrosis, Nat. Comm . 14(1): 2759, 2023. (* co-corresponding authors).
Valenca, S.S., B.E. Dong, E.M. Gordon, R.C. Sun, and C.M. Waters. ASK1 regulates bleomycin-induced pulmonary fibrosis, Am. J. Resp. Cell Mol. Biol. , 66(5): 484-496, 2022.
Trinkle, C.A., R.N. Broaddus, J.L. Sturgill, C.M. Waters, P.E. Morris. Simple, accurate calculation of mechanical power in Pressure Controlled Ventilation (PCV), Int. Care Med. Exp. 10: 22, 2022. PMCID: PMC9148680.
Immanuel, C.N., B. Teng, B.E. Dong, E.M. Gordon, J.A. Kennedy, C.L. Luellen, A. Schwingshackl, S.A. Cormier, E.A. Fitzpatrick, and C.M. Waters. Apoptosis signal regulating kinase-1 promotes inflammasome priming in macrophages, Am. J. Physiol. Lung Cell. Mol. Physiol. , 316: L418-L427, 2019.
Xiao, Z., J. Baudry, L. Cao, J. Huang, H. Chen, C.R. Yates, W. Li, C.M. Waters, J. Smith, L.D. Quarles. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis, J. Clin. Invest., 128(1): 157-174, 2018. PMCID: PMC5749530.
Wilhelm, K., E. Roan, A. Bada, and C.M. Waters. Kymographic imaging of the elastic modulus of epithelial cells during onset of migration, Biophys. J. 109 (10): 2051-2057, 2015. PMCID: PMC4656878.
Roan, E., K. Wilhelm, A. Bada, P.S. Makena, V.K. Gorantla, S.E. Sinclair, and C.M. Waters. Hyperoxia alters the mechanical properties of alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 302: L1235-L1241, 2012. Editorial Focus. PMCID: PMC3379045
Ghosh, M.C., P.S. Makena, J. Kennedy, B. Teng, C. Luellen, S.E. Sinclair, and C.M. Waters. A heteromeric molecular complex regulates the migration of lung alveolar epithelial cells during wound healing, Sci. Rep. 7(1): 2155, 2017. PMCID: PMC5438388.
Ghosh, M.C., V. Gorantla, P.S. Makena, C. Luellen, S.E. Sinclair, and C.M. Waters. Insulin like growth factor-1 stimulates differentiation of ATII cells to ATI-like cells through activation of Wnt5a. Am. J. Physiol. Lung Cell. Mol. Physiol. 305: L222-L228, 2013. PMCID: PMC3743013
Makena, P.S., V.K. Gorantla, M.C. Ghosh, L. Bezawada, L. Balazs, C. Luellen, K. Parthasarathi, C.M. Waters, and S.E. Sinclair. Deletion of apoptosis signal regulating kinase-1 prevents ventilator-induced lung injury in mice. Am. J. Resp. Cell Mol. Biol. 46: 461-469, 2012. PMCID: PMC3359950
Crosby, L.M., and C.M. Waters. Epithelial repair mechanisms in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 298: L715-L731, 2010. PMCID: PMC2886606.