- Augusta University
- Centers & Institutes
- Vascular Biology Center
- Masuko Ushio-Fukai - Research & Publications
Masuko Ushio-Fukai - Research & Publications
← Back to main
Research Interests
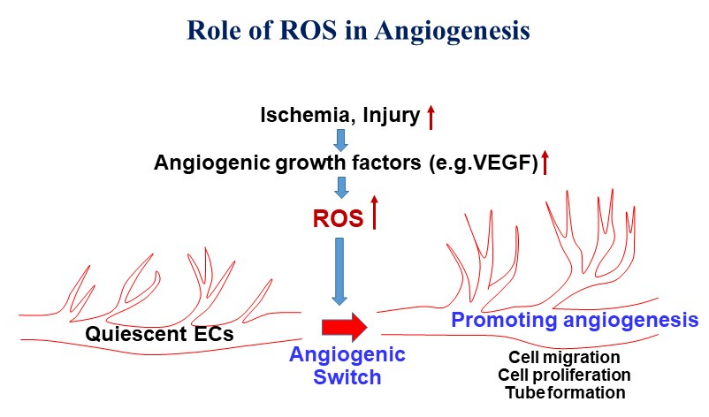
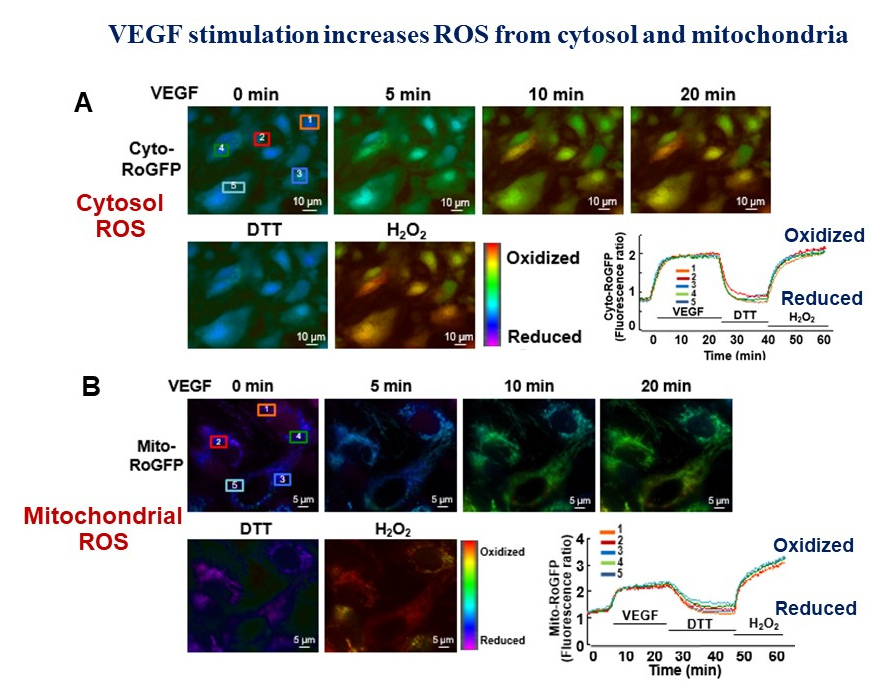
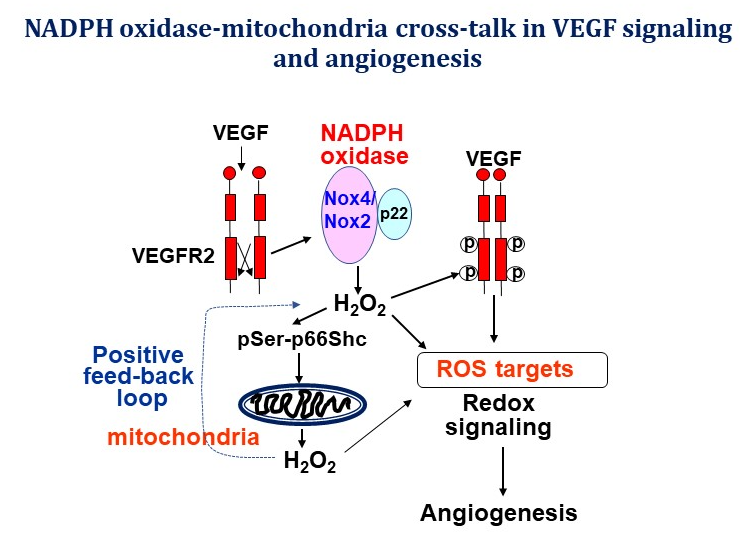
My research is focused on investigating the role of reactive oxygen species (ROS) and redox signaling in vascular biology and cardiovascular/metabolic disease, especially angiogenesis, a process of new blood vessel formation from quiescent endothelial cells (ECs), which is required for normal development, wound healing and neovascularization required for treatment of ischemic heart/limb disease. My laboratory is one of the first to demonstrate that endothelial H2O2 derived from NAPDH oxidase (NOX) is required for ischemia-induced neovascularization. Using yeast two-hybrid system, we identified IQGAP1, as a novel VEGF receptor type2 (VEGFR2)-binding scaffold protein, involved in compartmentalized ROS signaling at lamellipodial leading edge (see my invited review in Science STKE 2006), which promotes directional EC migration that drives angiogenesis. We also demonstrated that extracellular SOD (ecSOD)-derived H2O2 at caveolae/lipid rafts as well as “ROS-induced ROS release” orchestrated by NOX activation and mitochondria play a critical role in VEGFR2 signaling and angiogenesis. To determine the molecular mechanism of how highly diffusible H2O2 signal is sensed to promote angiogenesis, we employ the highly innovative biotin-conjugated Cys-OH trapping probe and redox proteomics approach to identify the ROS targets/sensors, “sulfenylated (Cys-OH formed) proteins, involved in angiogenesis. We also study the role of redox metal copper (Cu), an essential micronutrient, in angiogenesis and have demonstrated for the first time that Cysteine oxidation of Cu transport protein, CTR1, promotes VEGFR2 internalization and sustained signaling driving angiogenesis (Nature Cell Biology, 2022). My strong expertise in redox signaling and vascular biology will advance understanding of molecular mechanisms by which H2O2 signaling mediates vascular adaptation in response to ischemia and exercise while excess ROS (oxidative stress) contribute to many complications such as diabetes, aging, atherosclerosis, and tumor development. My lab uses molecular cell biology and biochemical approaches, cell imaging, protein-protein interaction and signal transduction analysis and various genetic mice models including CRISPR/Cas9-mediated knock-out and knock-in mice. Our long-term goal is to develop new therapeutic strategies for treatment of ischemic cardiovascular diseases, inflammaging, Alzheimer’s disease and cancer.
Research Projects
- Redox Signaling and Endothelial Cell Metabolism in Angiogenesis (retina, wound healing, hindlimb ischemia models).
- Mitochondria Dynamics and Autophagy in regulating Angiogenesis in Metabolic Diseases and inflammaging.
- Copper Transport Proteins in Angiogenesis and Diabetic Vascular Complications.
- Redox Regulation of Stem/Progenitor Cell Function and Bone Marrow Stem Cell Niche.
- Exosomes as mediator of redox signaling and angiogenesis
Spotlight Publications

$11.3 million NIH grant to help identify causes of vascular disease
$11.3 million NIH grant to help identify causes of vascular disease
Powerhouse-pruning protein may also aid new blood vessel growth in vascular disease
Powerhouse-pruning protein may also aid new blood vessel growth in vascular disease
Copper transporter potential new treatment target for cardiovascular disease
Copper transporter potential new treatment target for cardiovascular disease- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H Oxidase: Role in Cardiovascular Biology and Disease. Circulation Research. 2000; 86: 494-501. Cited by 2,948
- Ushio-Fukai M, Zafari Z, Fukui T, Ishizaka N, Griendling KK. p22phox is a Critical Component of the Superoxide-generating NADH/NADPH oxidase System and Regulates Angiotensin II-induced hypertrophy in Vascular Smooth Muscle Cells. Biol. Chem. 1996; 271: 23317-21. Cited by 792
- Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Science STKE. 2006; 349: 1-6. PMID: 16926363 (Invited review) Cited by 350
- Ushio-Fukai Redox Signaling in Angiogenesis: Role of NADPH oxidase. Cardiovascular Research. 2006; 71: 226-235. Cited by 449
- Fukai T and Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Disease. Antioxidants &Redox Signaling. 2011. 15: 1583-606. PMID: 214373702. Cited by 1,204
- Kim YM, Youn SW, Sudhahar V, Das A, Chandri R, Surenkhuu B, Kweon J, He L, Toth PT, Rehman J, Kitajewski J, Cho J, Fukai T, Ushio-Fukai M. Redox Regulation of Mitochondria Fission Protein Drp1 by Protein Disulfide Isomerase Limits Endothelial Senescence. Cell Reports 2018; 23:3565-3578. PMID: 29924999.
- Das A, Ash D, Kim YM, Sudhahar V, Hou Y, Hudson FZ, Stanfield BK, Fouda AY, Caldwell RB, McMenamin M, Fang X, Regan MR, Merrill BJ, Poole LB, Kaplan J, Fukai T, Ushio-Fukai M. Cysteine Oxidation of CTR1, the Cu uptake transporter, Drives VEGFR2 Signaling and Angiogenesis. Nature Cell Biology
Recent Publications
