- Augusta University
- Centers & Institutes
- Vascular Biology Center
- Tohru Fukai - Research & Publications
Tohru Fukai - Research & Publications
Research Interests
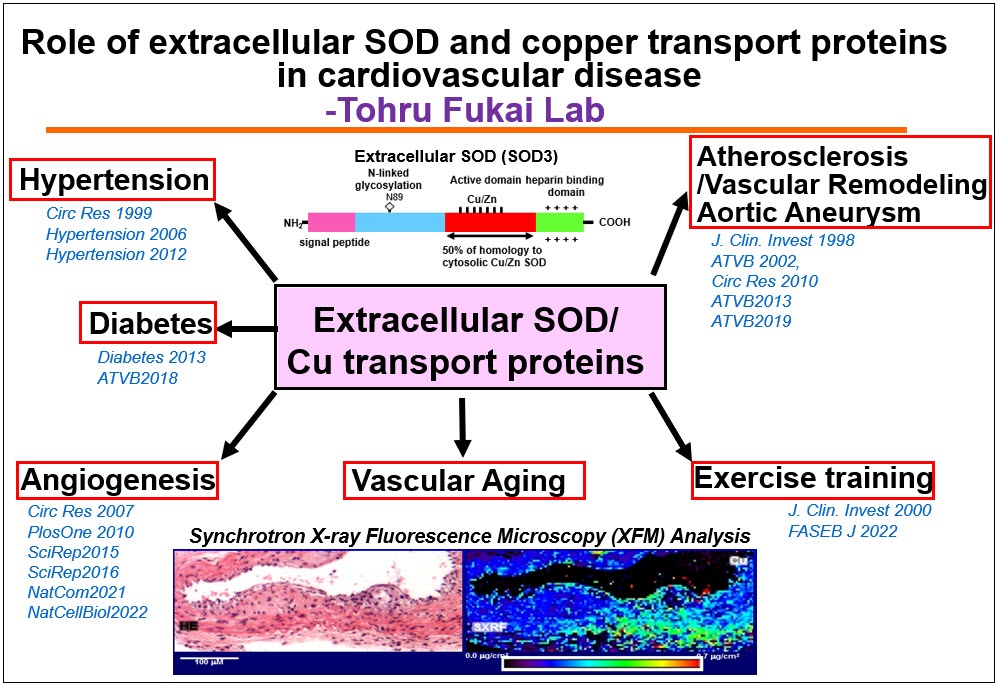
Oxidative stress plays an important role in the pathogenesis of
cardiovascular and metabolic disease such as hypertension, atherosclerosis
and diabetes. Our lab has been investigating a role of a major vascular
antioxidant enzyme extracellular SOD (ecSOD, SOD3)) and its regulator
copper (Cu) transport proteins in cardiovascular disease for almost two
decades. We have provided the first evidence that Cu uptake transporter
CTR1, Cu chaperone Atox1, and Cu transporting ATPase (ATP7A) play an
important role in hypertension, vascular remodeling, inflammatory
angiogenesis, atherosclerosis, and diabetes partly by regulating secretory
Cu enzymes, such as ecSOD and Lysyl oxidase. Unexpectedly, we also
found that Cu chaperone Atox1 also functions as a Cu-dependent
transcription factor to regulate cell proliferation and ROS/NFkB-dependent
inflammatory responses. Currently, we investigate the molecular
mechanisms of how oxidative stress and dysfunctional Cu metabolism
contribute to cardiovascular and metabolic diseases, such as hypertension,
atherosclerosis, diabetes, inflammaging, Alzheimer’s disease and cancer,
which will lead to development of novel therapy.
Research Projects
Extracellular Superoxide Dismutase (ecSOD, SOD3) and Cardiovascular disease
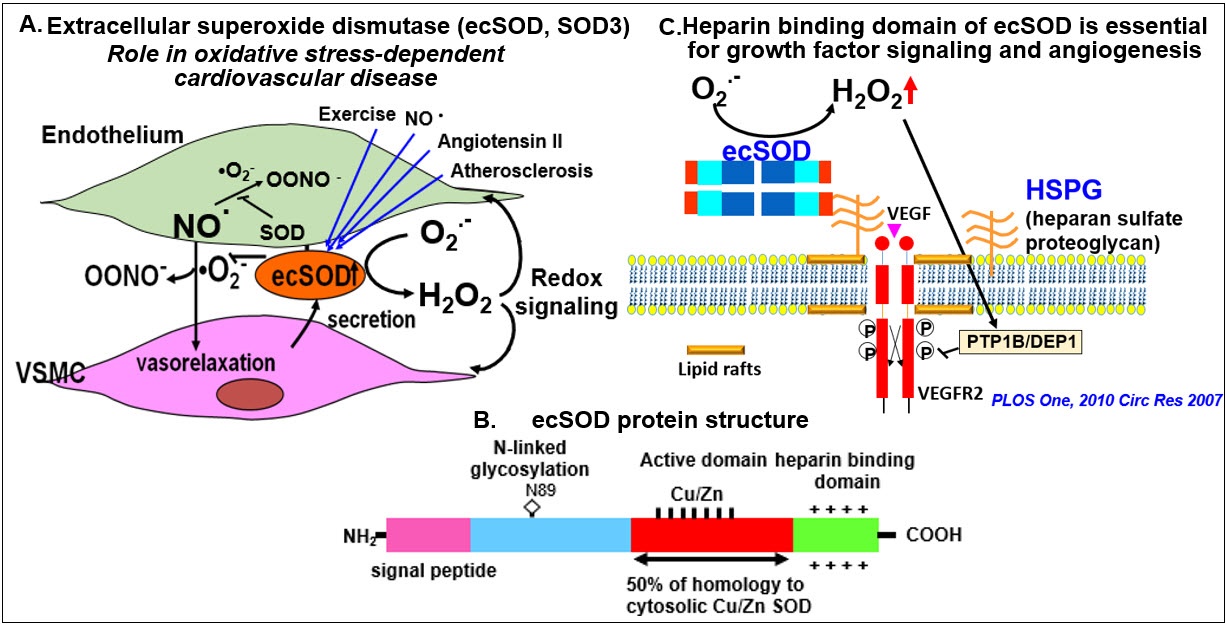 Extracellular SOD (ecSOD, SOD3) is a major extracellular antioxidant Cu-containing
enzyme highly expressed in the vasculature and synthesized by vascular smooth muscle
cells (VSMCs) and fibroblasts. It is secreted and anchored to the extracellular matrix
and endothelial cell surface through the heparin-binding domain (HBD). Because of
its extracellular location, ecSOD plays a major role in protecting against inactivation
of nitric oxide (NO) by superoxide (O2•-), thereby preventing endothelial dysfunction in oxidative stress–dependent cardiovascular
diseases. Furthermore, ecSOD catalyzes the dismutation of O2•- to H2O2 that functions as a signaling molecule in the vasculature via HBD. Using ecSOD knockout
mice, we demonstrated that ecSOD plays an important role in protecting against endothelial
dysfunction in hypertensin and diabetes as well as promoting angiogenesis. Most recently,
we demonstrated role of “exosomal” ecSOD in circulating plasma in exercise-induced
angiogenic effects in endothelial cells in type 2 diabetes. Given these findings,
we are studying role of ecSOD in vascular pathophysiology.
Extracellular SOD (ecSOD, SOD3) is a major extracellular antioxidant Cu-containing
enzyme highly expressed in the vasculature and synthesized by vascular smooth muscle
cells (VSMCs) and fibroblasts. It is secreted and anchored to the extracellular matrix
and endothelial cell surface through the heparin-binding domain (HBD). Because of
its extracellular location, ecSOD plays a major role in protecting against inactivation
of nitric oxide (NO) by superoxide (O2•-), thereby preventing endothelial dysfunction in oxidative stress–dependent cardiovascular
diseases. Furthermore, ecSOD catalyzes the dismutation of O2•- to H2O2 that functions as a signaling molecule in the vasculature via HBD. Using ecSOD knockout
mice, we demonstrated that ecSOD plays an important role in protecting against endothelial
dysfunction in hypertensin and diabetes as well as promoting angiogenesis. Most recently,
we demonstrated role of “exosomal” ecSOD in circulating plasma in exercise-induced
angiogenic effects in endothelial cells in type 2 diabetes. Given these findings,
we are studying role of ecSOD in vascular pathophysiology.
Cu transport proteins and Cardiovascular disease
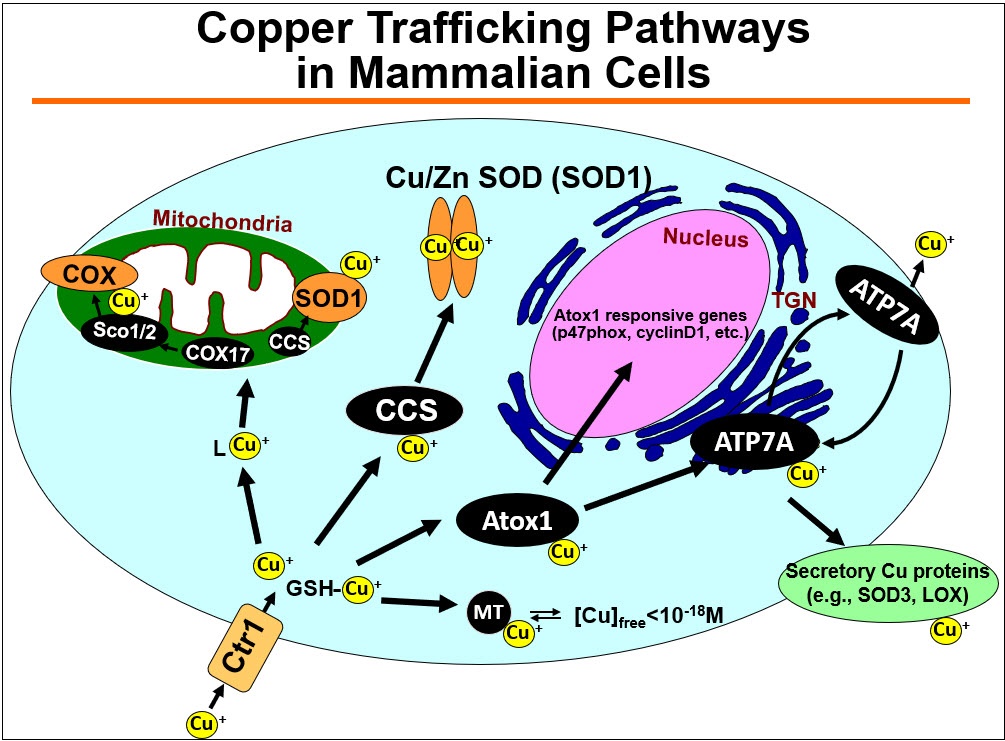
1) Cu transport proteins as key regulators of Cu containing enzymes (e.g. ecSOD, lysyl oxidase (LOX)) in cardiovascular disease
Cu, an essential trace element, serves as a cofactor of key Cu containing enzymes such as ecSOD and lysyl oxidase (LOX). Since Cu is toxic in excess, Cu homeostasis is tightly controlled by Cu transport proteins (Cu transporters/Cu chaperones) such as Cu uptake transporter CTR1, Cu chaperone Antioxidant1 (Atox1) that selectively transfer Cu to Cu transporter/exporter ATP7A. ATP7A localizes at the trans-Golgi network (TGN) to transport Cu to activate secretory Cu enzymes or translocates to the plasma membrane to secrete excess Cu. We previously demonstrated that these Cu transporters are involved in activating ecSOD and LOX which play an important role in oxidative stress-dependent endothelial dysfunction in hypertension and diabetes as well as vascular remodeling in response to injury or aortic aneurysm. Based on this, we examine role of vascular Cu transport proteins in vascular inflammatory disease and aging (Alzheimer disease) by regulating Cu containing enzymes.
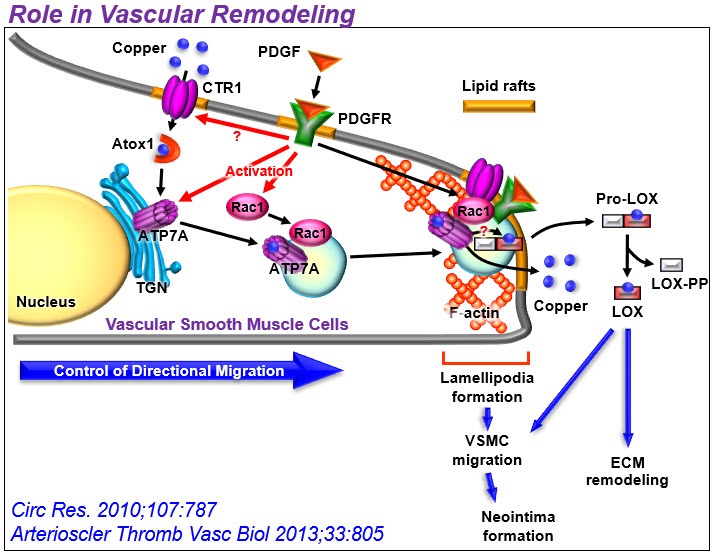
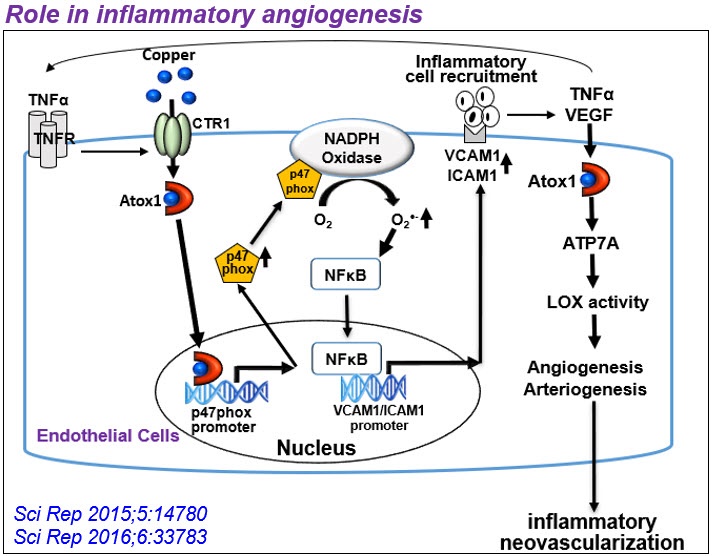
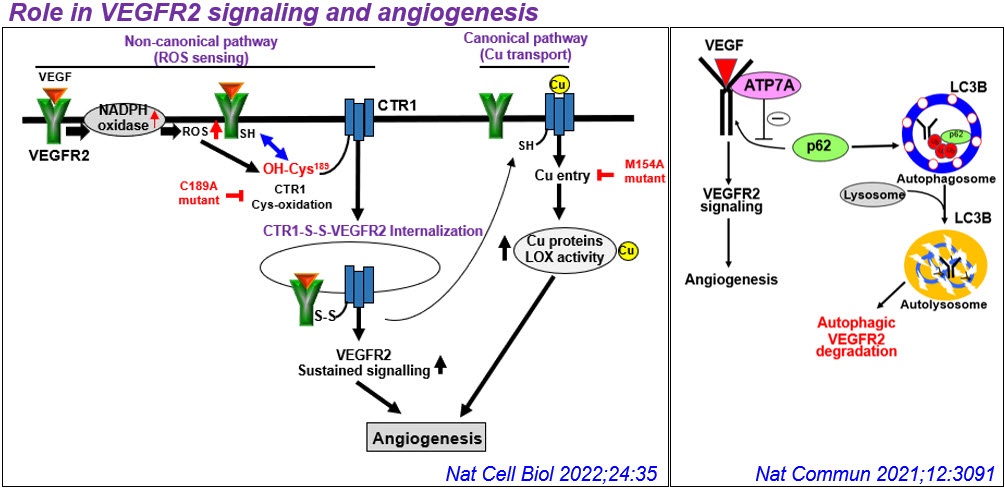
2) Unexpected role of Cu transport proteins in vascular inflammatory diseases. Cu has been implicated in vascular remodeling, diabetes, angiogenesis, aortic aneurysm, and atherosclerosis, but underlying mechanisms remain unclear. Unexpectedly, we found that cytosolic Cu chaperone Atox1 is translocated to the nucleus to function as a Cu-dependent transcription factor promoting cell proliferation and ROS/NFkB-dependent inflammatory gene expression under inflammation such as atherosclerosis. Furthermore, we found that Cu transporter ATP7A is translocated from TGN to lipid rafts/lamellipodial leading edge in response to PDGF to promote VSMC migration in part by regulating Rac1 and LOX activity. We also demonstrated protective role of vascular ATP7A against aortic aneurysm and Cu-independent role of endothelial ATP7A and CTR1 in VEGFR2 signaling angiogenesis. Based on these findings, our lab extensively investigates role of vascular and inflammatory Cu transport proteins in growth factor and redox signaling in vascular disease.
Spotlight Publications

$11.3 million NIH grant to help identify causes of vascular disease
$11.3 million NIH grant to help identify causes of vascular disease
Powerhouse-pruning protein may also aid new blood vessel growth in vascular disease
Powerhouse-pruning protein may also aid new blood vessel growth in vascular disease
Copper transporter potential new treatment target for cardiovascular disease
Copper transporter potential new treatment target for cardiovascular disease
- Fukai T# and Ushio-Fukai M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Disease. Antioxidants &Redox Signaling. 2011. 15: 1583-606. Cited by 1,204
- Fukai T#, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease (invited review). Am J Physiol Cell Physiol. 2018. 315:C186-C201.
- Ash D, Sudhahar V, Youn SW, Okur MN, O‘Brien JP, McMenamin M, Fang X, Hou Y, Kaplan JH, Fukai T#, Ushio-Fukai M#. ATP7A promotes angiogenesis by limiting autophagic degradation of VEGFR2. Nat Commun 2021;12(1):3091. #:co-senior/corresponding author
- Das A, Ash D, Kim YM, Sudhahar V, Hou Y, Hudson FZ, Stanfield BK, Fouda AY, Caldwell RB, McMenamin M, Fang X, Regan MR, Merrill BJ, Poole LB, Kaplan J, Fukai T#, Ushio-Fukai M#. Cysteine Oxidation of SLC31A1/CTR1, the Cu uptake transporter, Drives VEGFR2 Signaling and Angiogenesis. Nat Cell Biol. 2022;24(1):35-50. #:co-senior/corresponding author
- Abdelsaid K, Sudhahar V, Harris RA, Das A, Youn SW, Liu Y, McMenamin M, Hou Y, Fulton D, Hamrick MW, Tang Y, Fukai T#, Ushio-Fukai M#. Exercise improves angiogenic function of circulating exosomes in type 2 diabetes: Role of exosomal SOD3. FASEB J. 2022 Mar;36(3):e22177. #:co-senior/corresponding author
Recent Publications
