Graydon B. Gonsalvez, PhD

Professor
Department of Cellular Biology & Anatomy
Office: R&E Building, CB2917
Phone: 706-721-1756
Fax: 706-721-6120
ggonsalvez@augusta.edu
Jump to: Education & Post Doctoral Training Current Funding Lab Members Publications
Education & Post Doctoral Training
Education
94-98 BA Carthage College, Kenosha, WI
98-04 PhD. Medical College of Wisconsin, Milwaukee, WI
Post Doctoral Training
04-07 Case Western Reserve University, Cleveland, OH
07-09 University of North Carolina, Chapel Hill, NC
Current Funding
The Gonsalvez lab is currently funded by an R01 grant from the National Institutes of General Medical Sciences (2013-2018). Past funding include a grant from the American Cancer Society and an intramural grant from Augusta University.
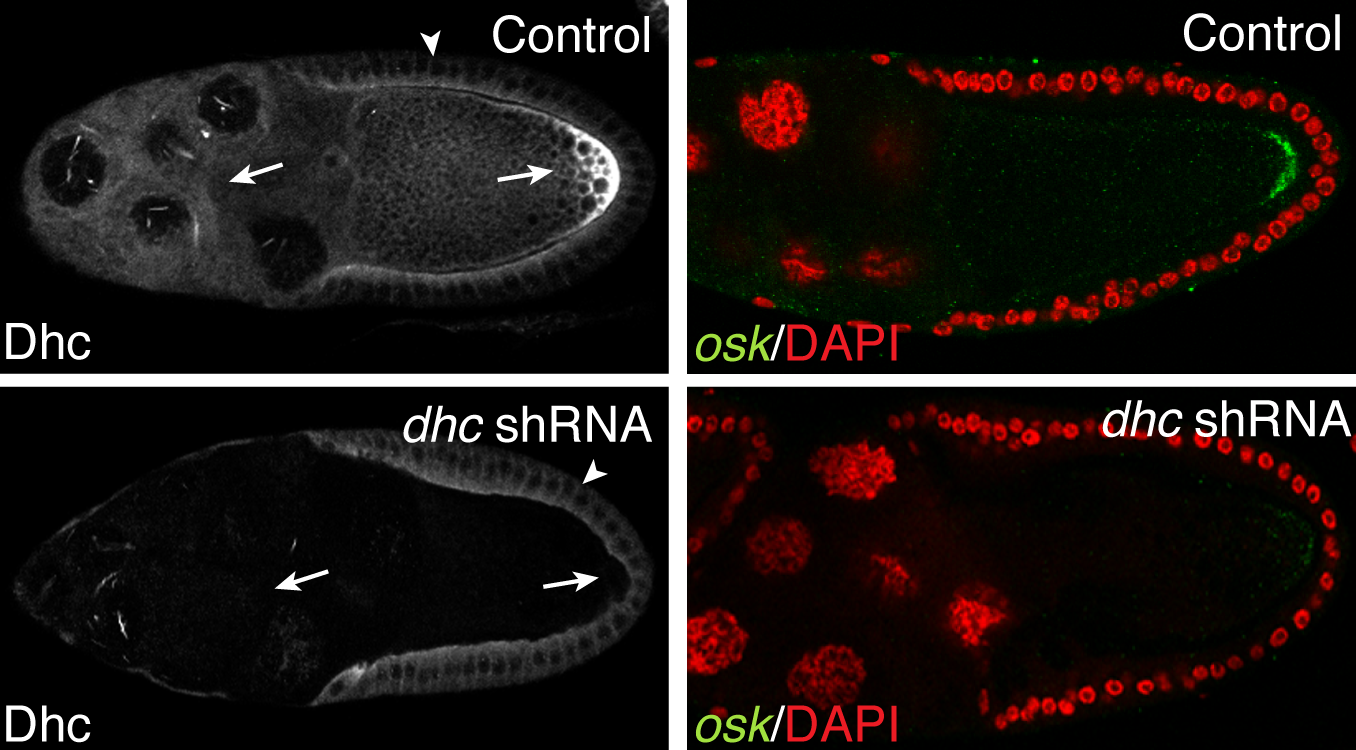
- The top left panel in the above figure shows a control egg chamber. The motor, Dynein, as well as oskar mRNA are localized at the posterior pole. The bottom panel represents an egg chamber in which Dynein has been depleted. This results in the delocalization of oskar mRNA.
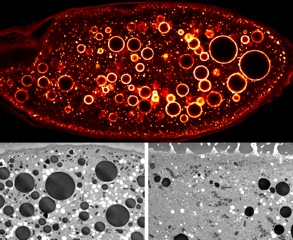
- The top middle panel of this figure illustrates the large endocytic intermediates that are observed when Dynein function is compromised (large circles). The bottom panel show electron microscopy images from control (left) and Dynein inhibited (right) egg chambers.
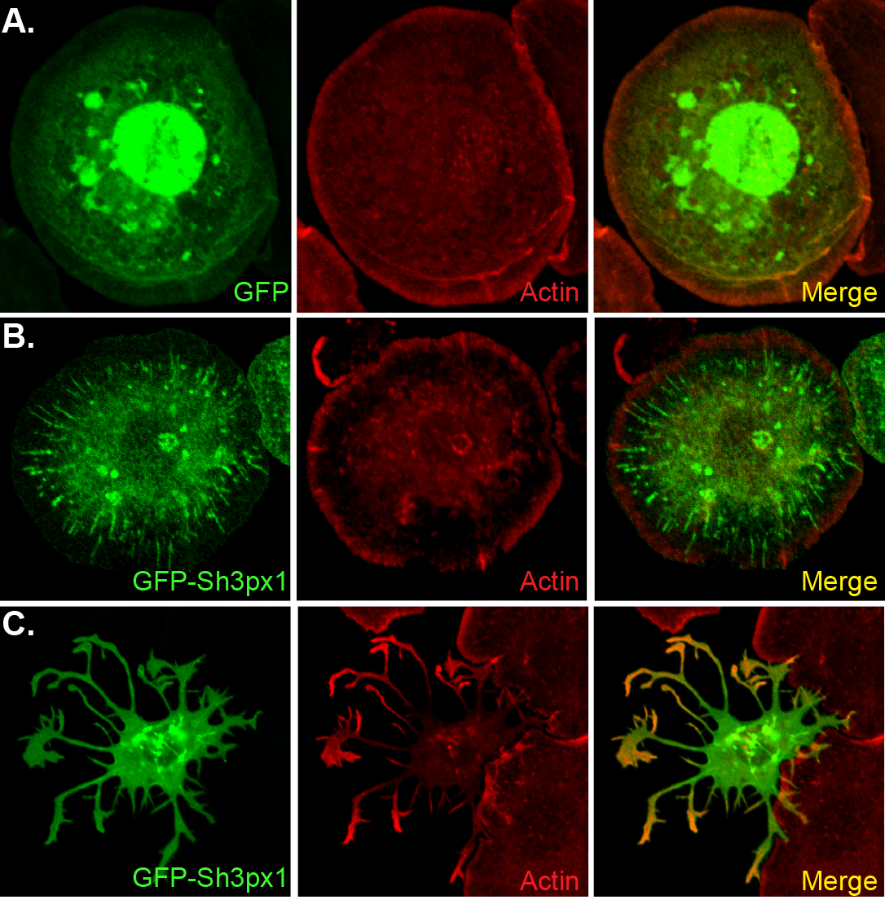
- The top right panel of this figure shows a control Drosophila S2 cells that is expressing GFP. In the middle panel, the cell is over-expressing GFP-Sh3px1. This cell has formed long tubules. The bottom panel shows a cell over-expressing GFP-Sh3px1. This cell has formed long protrusions.
Endocytosis. Endocytosis is the mechanism by which cargoes are internalized and sorted within the cell. In addition to mRNA localization, endocytic trafficking also contributes to the establishment of cell polarity. Our lab has recently shown that the Dynein motor complex performs an essential function in endocytic maturation. When Dynein is inhibited, internalized cargo is trapped in enlarged endocytic vesicles (larges circles in the top panel). Our current efforts are focused on determining the precise mechanism by which Dynein functions in the endocytic pathway.
In order for cargo to be trafficked through the endocytic pathway, the membrane on endocytic vesicles has to become curved. This is a thermodynamically unfavorable process. Protein containing a domain known as a BAR domain are known to aid in this process. BAR domain proteins generate either membrane tubules or protrusions when over-expressed in cells. Our lab is currently studying the functions of a protein called Sh3px1. This protein contains a unique kind of BAR domain. When Sh3px1 is over-expressed in cells, it dramatically alters cell morphology. Some cells form long tubules, whereas other cells form long membrane protrusions. We are currently exploring the role of Sh3xp1 in development and tissue morphogenesis.
Long term goals: Defects in cell polarity are associated with several diseases including cancer, polycystic kidney disease and Charcot-Marie-Tooth disorder. The long-term goal of our research is to design better therapies to treat these debilitating diseases by determining how healthy cells establish polarity, and by understanding the way in which defective molecular pathways contribute to the disease state.
Approaches: We use a variety of tools such as genetics, cell biology and biochemistry to test our hypotheses. Numerous molecular techniques such as RT-PCR, western blotting, immunoprecipitation and in situ hybridization are routinely used. Studies are performed using Drosophila, Drosophila cell lines and mammalian cell lines.
Current Members of the Lab
Phylicia Allen
- Graduate Student
Jessica Pride
- Graduate Student
Hannah Neiswender
- Research Associate
Publications
Activation of receptor-independent fluid-phase pinocytosis promotes foamy monocyte
formation in atherosclerotic mice.
Ahn W, Burnett FN, Wojnar-Lason K, Doja J, Sreekumar A, Ghoshal P, Singla B, Gonsalvez G, Harris RA, Wang X, Miano JM, Csányi G.Redox Biol. 2024 Dec;78:103423. doi: 10.1016/j.redox.2024.103423. Epub 2024 Nov 6.PMID: 39615283
Sigma 1 Receptor Contributes to Astrocyte-Mediated Retinal Ganglion Cell Protection.
Zhao J, Gonsalvez GB, Mysona BA, Smith SB, Bollinger KE.Invest Ophthalmol Vis Sci. 2022 Feb 1;63(2):1. doi: 10.1167/iovs.63.2.1.PMID: 35103752